LifePort announced that it has signed an agreement with EpiGuard (Fredrikstad, Norway) to distribute the EpiShuttle in the United States and Canada. LifePort will be leveraging expertise and market penetration within air ambulance and broader aerospace while partnering with EpiGuard’s proven patient isolation solution to support the fight against Covid-19.

LifePort will design, engineer, and manufacture components that allow the EpiShuttle to be secured in a wide variety of transportation vehicles. Aviation or ground applications are designed to enable a seamless interface while providing a secure method of attaching the EpiShuttle in the vehicle. Through LifePort, the EpiShuttle is currently registered with both the FDA and Health Canada, and will be accompanied by a structural engineering report for the interface brackets.
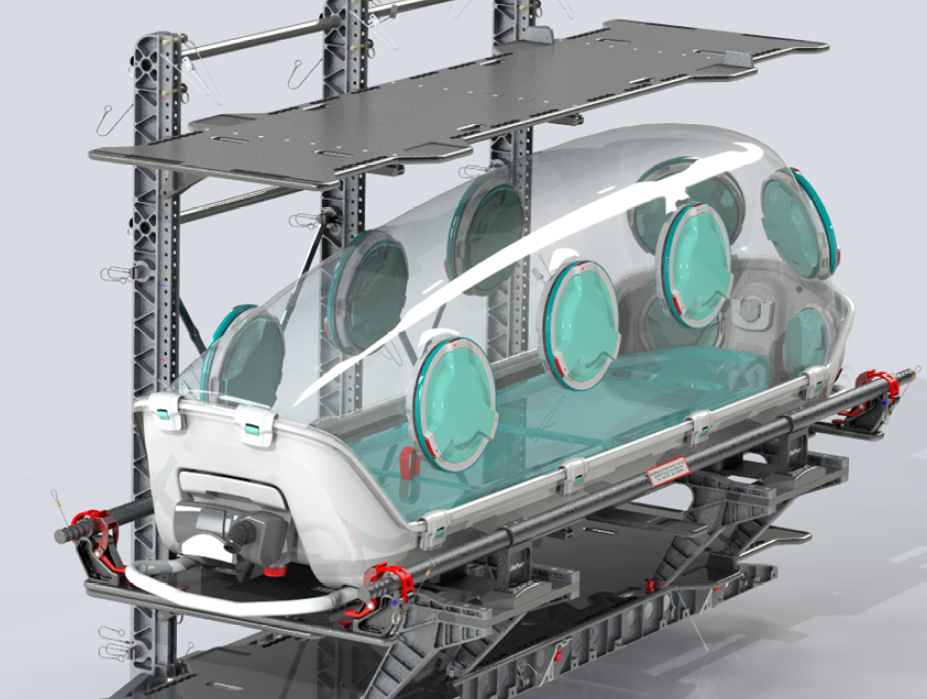
The EpiShuttle by EpiGuard is a single-patient isolation and transport system, designed to provide maximum patient safety and comfort while allowing critical care and treatment to be performed. The EpiShuttle is designed to protect both the environment and patient from contamination.
“Both crew and aircraft are protected against contamination with the patient placed in an EpiShuttle,” said CEO of EpiGuard, Ellen Cathrine Andersen. “At the same time, the patient can receive lifesaving treatment. An ambulance usually requires two to four hours of disinfection between every contagious transport, and with an entire helicopter, it takes almost a day. Because the EpiShuttle is underpressurized and sealed it reduces the need for disinfection, saving valuable time and resources. Saving one and protecting everyone, the EpiShuttle is ready to deploy in North America.”
“I am very excited to reach an agreement that leverages the sales, technical and manufacturing capabilities of EpiGuard and LifePort; which will result in increased product availability to the U.S. and Canadian markets,” said Jason Darley, CEO of LifePort. “I have no doubt that our partnership will increase the safety and functionality of our products in the transport of infected or potentially infected patients.”
“We chose to distribute the EpiShuttle in the U.S. and Canada through LifePort because of their strong market presence, relevant users’ proven confidence and most of all because of the depth of knowledge and expertise we find at LifePort,” said Andersen. “We are certain that LifePorts’ understanding of the functionalities and benefits of the EpiShuttle, combined with their knowledge of the North American market, makes for a great partnership.
“The EpiShuttle is already in service with the Royal British Air Force, the Royal Norwegian Air Force and the Royal Danish Air Force, and with LifePort on board, I am confident that the EpiShuttle will save and protect North-Americans as well,” Andersen added.